Official data on the efficacy rates of Sinovac‘s coronavirus vaccine (CoronaVac) are kept from the public due to a confidentiality deal, according to a member of the Department of Health’s Technical Advisory Group.
Infectious diseases expert Dr. Edsel Salvana made this explanation in a Facebook post in response to a query about the missing data on the China-made vaccine.
The main post was about Salvana’s decision to receive CoronaVac and convincing other health workers to do the same.
It was shared on February 26, ahead of the arrival of any vaccine brand in the country on February 28.
Salvana was among the health officials who took the jab during the launch of the government’s vaccination program on March 1.
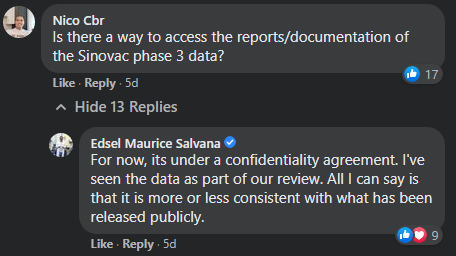
In the comments section of this post, Nico Cabrera, a board-certified internal medicine specialist and immunocompromised host/transplant infectious disease fellow at Stanford University, asked Salvana if there is a way for the public to see the results of CoronaVac’s phase three clinical trial tests.
Salvana replied that it is not possible, citing a confidentiality agreement.
“For now, it’s under a confidentiality agreement. I’ve seen the data as part of our review. All I can say is that it is more or less consistent with what has been released publicly,” he said.
Cabrera then asked if this is a standard or a rule for pharmaceutical products submitted to the country’s Food and Drug Administration.
Salvana pointed out that the confidentiality is “not unusual.”
“Yes. This is an EUA and there are trade secrets. It’s not unusual,” the physician replied.

Sinovac skepticism
Health workers and other Filipinos are hesitant to receive CoronaVac mainly because of the missing phase 3 trial results and FDA’s decision not to recommend the vaccine to medical front-liners.
READ: Vaccine distrust worries as FDA says Sinovac COVID-19 jab not recommended to medical frontliners
“Most PGH residents and fellows I know are refusing Sinovac, some out of serious concerns about efficacy, safety. But many out of principle, in protest over the lack of transparency over data for which EUA was granted,” one Twitter user said.
Statistician Peter Cayton also asked Sinovac’s efficacy for other coronavirus variants.
“Wait… saang scientific paper po nakasaad ang efficacy ng Sinovac sa variants? Wala lang po akong mahanap,” Cayton asked.
Dr. Jonas del Rosario of the Philippine General Hospital, which was the first institution to receive a Sinovac shot, previously said that only 12% or around 2,000 employees are willing to get inoculated with it.
Aside from CoronaVac, AstraZeneca also arrived in the Philippines on March 4.
So far, only COVID-19 vaccines from pharmaceutical companies Sinovac, Pfizer-BioNTech and AstraZeneca have been FDA-approved for emergency use.
A different issue with non-Western vaccines?
In the same comments section of Salvana’s post, Cabrera further inquired if the requirements for approval with the United States’ FDA is different, citing the latter’s approval of vaccines with publicly-available data.
Salvana responded and claimed that it’s an “issue” with vaccines from non-Western pharmaceutical companies.
Pfizer-BioNTech is from a joint venture of the US-based Pfizer and the German firm BioNTech. AstraZeneca, meanwhile, is developed by Oxford University and AstraZeneca.
“That’s an issue with non-Western companies. The EUA is also a new animal and is different from regular approval,” he said.
In the same conversation thread, Salvana further expounded the Philippine FDA’s process for approving non-regulated vaccines.
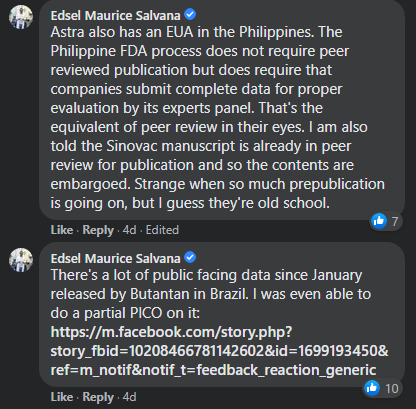
“The Philippine FDA process does not require peer reviewed publication but does require that companies submit complete data for proper evaluation by its experts panel. That’s the equivalent of peer review in their eyes,” he said.
“I am also told the Sinovac manuscript is already in peer review for publication and so the contents are embargoed. Strange when so much prepublication is going on, but I guess they’re old school,” he added.