
Phase 2 of A3.1 vaccination program starts rolling out in 17 more locations in the Philippines
When the news broke out that children with comorbidities can get vaccinated, Paul Vincent Lim immediately registered his son for vaccination.
As early as 9 a.m., Lim and his 15-year-old son were already at the SM Megamall Mega Trade Hall, waiting to get inoculated.
“The benefits outweigh the risk. The moment I knew that he was eligible for inoculation, I did not hesitate to register his name. His pedia also approved of it so we pushed through with it,” said Lim.
Lim’s son is just one of the 1.2 million children aged 12 to 17 with comorbidities in the country. And last Thursday, he got his son the ultimate protection against this dreaded disease– COVID-19 vaccines.
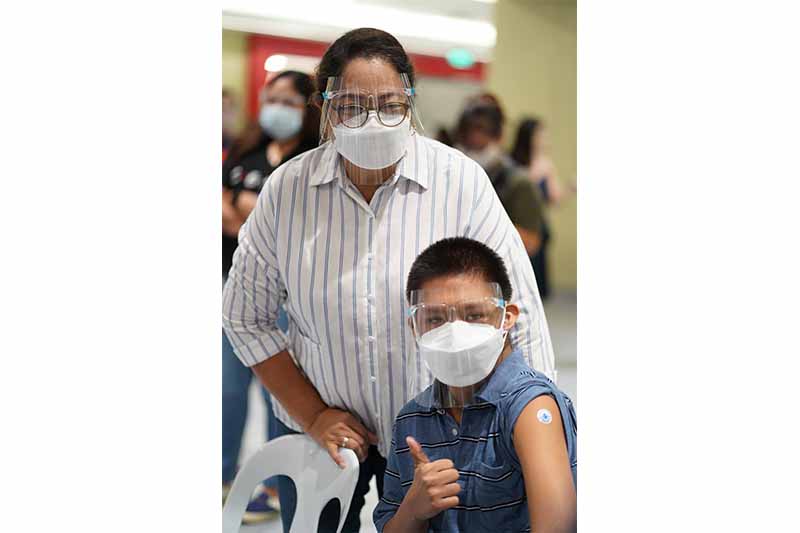
In cooperation with the Department of Health (DOH), the Inter-Agency Task Force and National Task Force Against COVID, National Vaccination Operation Center (NVOC), and Mandaluyong City LGU, SM Supermalls opened the SM Megamall Pediatric Vaccination Center to Filipino children who have underlying medical conditions on Thursday, October 21.

“At SM, we remain committed to providing accessible and convenient vaccination areas to our communities and beyond. Now that the roll out of the inoculation of minors with preexisting conditions have started, we will continue to lend a helping hand to the government and give them the necessary support to boost the country’s vaccination drive,” SM Supermalls President Steven Tan said.
Around 120 individuals who belong to the A3.1 category went to the mega vaccination center to get inoculated against COVID-19. SM Megamall is part of the 17 locations and the only mall venue where Phase 2 of the program is being rolled out.
Prior to the opening, Mandaluyong City Mayor Menchie Abalos said that they’ve prepared really well to ensure the safety of the kids.
“Vaccinating children can be quite challenging. We had to make some modifications in the vaccination center like putting up dividers where the kids will be getting jabbed. Also, we have prepared well for emergencies. Our team conducted a simulation yesterday to ensure that we can respond to emergency situations immediately,” Abalos noted.
The MandaVax site has currently received around 7000 registrations, and around a thousand of it are children with comorbidities.

The SM Megamall Pediatric Vaccination Center will cater to Mandaluyong residents at the moment, however, Abalos mentioned that non-Mandaluyong residents who work in the city and have children with comorbidities will soon be accommodated.
The phased approach to vaccinating the A3.1 category began last October 15. DOH emphasized that the rollout of inoculation among children and minors was needed to manage their comorbidity risks.
“It’s been a while since developed countries have started administering pediatric vaccines, and we believe that it’s high time for us to start this already. We chose SM Megamall as one of our venue partners for the second phase of the A3.1 vaccination roll out because we know that they can provide vast, safe, and convenient spaces, ideal for vaccination. We are grateful to SM Supermalls and SM Megamall for opening their vaccination centers to us,” said Chief Local Health Support Division and Vaccine Cluster Head, Dra. Amelia Medina.
To date, SM Supermalls has successfully administered over 4.5M jabs to Filipinos under its multi-mall vaccination program that is aimed at curbing the spread of the COVID-19.
With its long-standing commitment to ensuring the health and safety of the public, SM continues to provide not just a #SafeMalling experience to shoppers, but also a convenient and accessible venue where their communities can get vaccinated.
Parents and guardians can pre-register their children via the MandaVax site using their household code.
The LGU added that those who may have questions regarding the vaccination of minors may call the following MANDAVAX hotlines: 0917-17-62632, 0917-18-62632, 0917-67-62632, 0968-609-5405, 0915-497-2946, 0919-524-5715, 8532-5001 loc 471 to 480
The DOH also stressed the following requirements and processes to be observed at the vaccination areas:
- Informed consent of parents, as well as approval from children who will receive vaccines themselves;
- Medical certificate from doctors indicating comorbidities of the child/minor vaccinee; and,
- In far-flung or Geographically-Isolated and Disadvantaged Areas (GIDAS), a clearance from on-site trained physicians guided by a checklist from the Pediatric Infectious Disease Society of the Philippines is to be provided.









