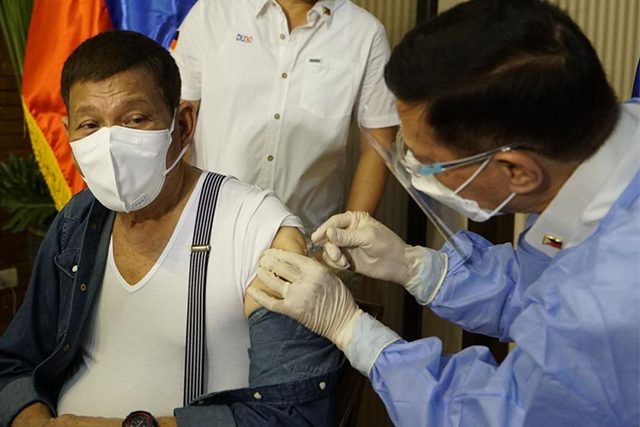
President Rodrigo Duterte received his first dose of Sinopharm’s vaccine against COVID-19 despite it being given a “compassionate special permit” for his close-in security personnel only.
Sinopharm has also yet to be granted an emergency use approval by the country’s Food and Drug Administration.
In a Facebook Live aired on Monday night, May 3, Duterte was shown being administered with a dose of the vaccine from China by Health Secretary Francisco Duque III. The 76-year-old president was also with his former aide-turned-senator.
READ: Duterte receives first dose of Sinopharm’s COVID-19 vaccine
“This confirms that (President Duterte) received his first dose tonight of the Sinopharm anti-COVID-19 vaccine,” said presidential spokesman Harry Roque.
This was the same vaccine brand the Presidential Security Group personnel, which are tasked to provide close-in security and escort to the president, had received as early as September 2020.
However, unlike Duterte’s case, the FDA issued a compassionate special permit (CSP) for use of 10,000 doses of Sinopharm’s COVID-19 jab to be given to the PSG.
Reviewing the CSP
The CSP was issued last February 2021, months after the illegal vaccination took place.
Despite the timing, FDA Director Eric Domingo defended the issuance of the special license saying that the PSG applied and complied with the requirements for it.
“The PSG applied and complied with all requirements for the CSP. It was granted yesterday and the PSG Hospital takes full responsibility for the vaccines and will report utilization and outcomes to [us],” Domingo was quoted as saying in Philstar.com report.
When asked if it is retroactive or taking effect prior to being enacted, Domingo responded to Philstar.com in February:
“Not really. Because CSP is used to import the drug or the vaccine.”
As of writing, Sinopharm’s EUA is still pending before the FDA. Without it, the vaccination is considered illegal under Republic Act No. 9711 or the FDA Act of 2009.
Only COVID-19 vaccines that were granted with the EUA can be used within the duration of the pandemic.
Aside from the PSG, Ramon “Mon” Tulfo, the government’s special envoy for public diplomacy to China, claimed that he and other government officials have also received Sinopharm last year.
Criticisms vs Sinopharm’s vaccine
The keyword “Sinopharm” trended on Twitter on Monday evening as Filipinos denounced Duterte’s inoculation with the unregistered vaccine.
“Walang EUA yung bakuna (meaning illegal) tapos ipinagmamagaling niyo pa? Ang lala niyo po,” one user said.
“We all know Malacañang smuggled Sinopharm vaccines way back, and by the way, officially the Philippines has ZERO orders of Sinopharm. The FDA hasn’t even given Sinopharm EUA yet. All I see here are three idiots plundering this country dry,” another user wrote.
“Yep, what a snapshot of pandemic governance in the Philippines,” a user commented.
Columnist Manuel Quezon III also raised the question on why the government preferred Sinovac’s CoronaVac instead of Sinopharm’s COVID-19 jab.
“Reporters: the unanswered question is why Sinopharm was preferred for the President while Sinovac is the one the government bet on for the public,” he said.
The national government has been promoting the use of Sinovac’s CoronaVac to the public.
A total of 600,000 doses of CoronaVac arrived in the Philippines last February 28 days after it was given the EUA.
The vaccination in the country started March 1.









