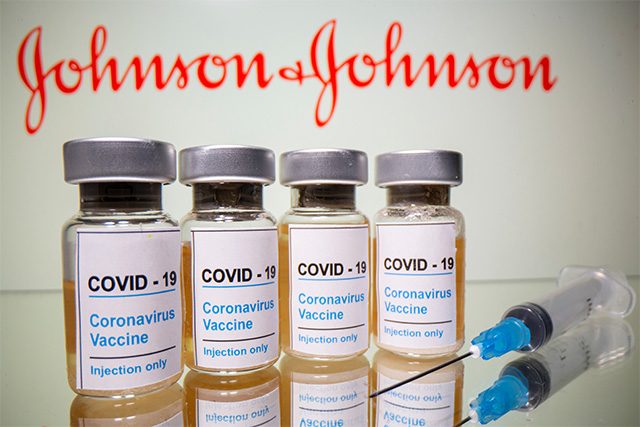
MANILA — Drugmaker Johnson & Johnson has filed its application for emergency use authorization of its COVID-19 vaccine in the Philippines, the country’s Food and Drug Administration (FDA) chief said on Monday.
J&J’s documents, which were submitted on Wednesday, are now under evaluation, FDA Director General Rolando Enrique Domingo told reporters. J&J, which has a single-shot coronavirus vaccine, is the seventh vaccine maker to seek approval in the Philippines. —Reporting by Neil Jerome Morales; Editing by Martin Petty