Elmera Balaba tells News5 the good news that her 10-year old child was one of the hundreds of thousands of children and public school pupils who completed the prescribed three-dose regime of the Dengvaxia vaccine, at no cost, for dengue. But the positivity has proved to be short-lived: The efficacy of the vaccine was lately discovered to be hounded by a gap – a serious gap for her child and for the other children inoculated in the mass vaccination campaign.
The community and school-based mass vaccination was launched in April 2016 – just before the conduct of the May national election.
To be given in three doses at six-month intervals, Dengvaxia was expected to help reduce morbidity and mortality from the mosquito-borne dengue hemorrhagic fever.
Comes now the bummer: Referencing up to six years of clinical data, Sanofi disclosed the result of its analysis that, “for those not previously infected by dengue virus, more cases of severe disease could occur following vaccination upon a subsequent dengue infection.”
In effect, Sanofi was saying that its dengue vaccine mostly helps people who had been previously infected by the virus.
“We are working with health authorities to ensure that prescribers, vaccinators and patients are fully informed of the new findings,” said Dr. Su-Peing Ng, Global Medical Head of Sanofi.
This has prompted concerned parents and other stakeholders to ask: How to do right by these children who were inoculated with Dengvaxia without first being queried if they have not had a previous brush with dengue?
DOH suspends program
On Friday this week, the Department of Health announced the suspension of the vaccination program after the vaccine’s manufacturer, French pharmaceutical giant Sanofi Pasteur, revealed two days previously that its landmark dengue vaccine could worsen the disease among those who were not previously infected by the virus.
Click and watch this related video report from News5, below:
For its part, the Department of Education said on Saturday it will monitor the condition of vaccinated students and “closely coordinate with the Department of Health in ongoing surveillance of students who received the dengue vaccine, as the health and safety of our learners are of principal importance. As a stakeholder, DepEd will likewise be actively participating in the review and consultations of DOH on the dengue vaccination program.”
Health Secretary Francisco Duque III assured the public that the individuals who received dengue vaccine, whether previously infected or not, are expected to get a 30-month protection from the virus from first dose.
Big question mark
Duque was quick to clarify that no cases of “severe dengue infection” have been reported yet out of least 733,713 children who were given the vaccine starting in 2016, but he added that DOH wants to clarify with Sanofi just what constitutes a “severe disease,” the term that it used in the disclosure.
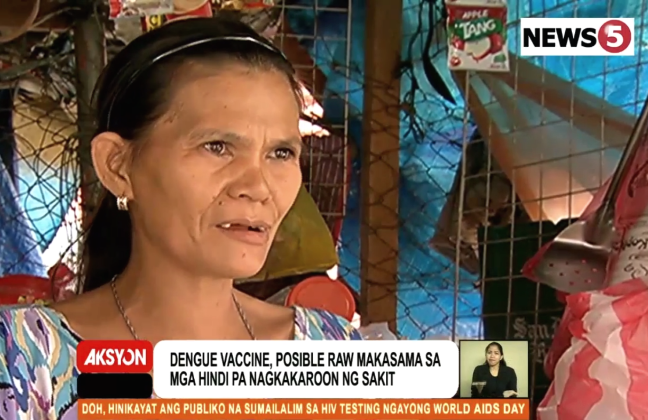
For Elmera Balaba and her husband, the news raised a big question mark: “Bakit ganun naman tinutusok nila sa anak ko, na ganun pala ang resulta eh parang ginagawa nilang laruan at ineeksperimentuhan ang anak ko? Iba kasi ang inexplain nila sa amin, maligtas yung bata (What did they do to my child that it had come to this? It’s as if they had toyed or experimented on our child).”
“Umaasa ako sa paliwanag, DOH yun eh. Tiwala ako sa kanila, para sa kaligtasan ng anak ko, na hindi na siya magkadengue, mahirap magkasakit ng dengue ah (I had relied on the explanation, after all, it was DOH, that the child would be protected, because getting sick with dengue is not to be taken lightly),” she added, her face painted in dismay.
Strict monitoring, surveillance
About 700,000 individuals received at least one dose of the vaccine. DOH records show that an estimated 789,010 units of Dengvaxia have yet to be used, but are about to expire in May 2018.
For her part, former health chief Dr. Janette Garin indicated that DOH has established a “strict monitoring and surveillance system for adverse events and side effects following immunization.”
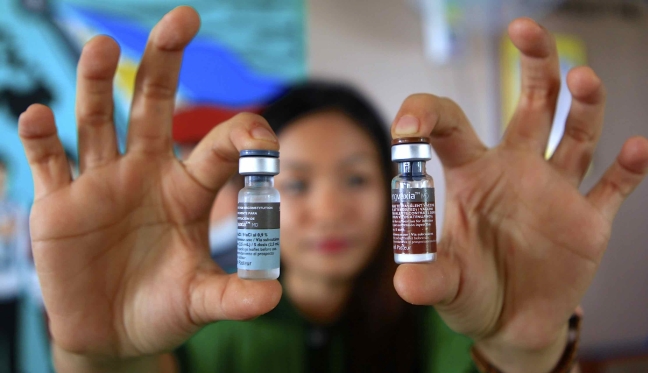
Dr. Garin was at the forefront of the program’s launch, initially aimed at Grade Four public school pupils in three of the regions with the highest prevalence of dengue – the National Capital Region, Central Luzon and part of Southern Luzon, Calabarzon. She pointed out that the Philippines was the first country to implement the dengue mass vaccination using the government’s public health infrastructure and the public school system.
Bummer backlash
But Sanofi’s recent Aha! Moment regarding the conditional efficacy has proved to be a major bummer. In reaction, Dante Jimenez, who chairs the anti-crime group Volunteers Against Crime and Corruption, asked DOH to set up “centers” in provinces where parents whose children have received Dengvaxia may file their complaints.
“This is even worse than any heinous crimes,” Jimenez said.
For his part, Senator JV Ejercito, chair of the Senate Committee on Health and Demography, has calendared a legislative inquiry in January next year.
Haphazard, not ready
Senator Joel Villanueva called for accountability to be exacted from Sanofi as well as Department of Health officials responsible for “haphazardly allowing the vaccine to be administered without extensive due diligence on the effects and without waiting for the results of comprehensive clinical trial.”
Even more scathingly, Senator Richard Gordon, chairman of the Senate Committee on Accountability of Officers and Investigations, or Blue Ribbon, revealed that an official of the World Health Organization (WHO) had earlier warned, at the time Dengvaxia was procured by the administration of then President Benigno Aquino III, that the vaccine had not received pre-qualification approval.
See also:
DENGVAXIA BACKLASH | Experts had told PNoy govt of risks from dengue vaccine – Gordon
This means the vaccine was not yet ready for distribution, explained Senator Gordon. “Now we have the evidence on that.”
Gordon elaborated: “Before we conducted an investigation on the anomalous procurement of this vaccine, we talked to several health experts and they told us that they had already warned government long before about the possible adverse effect of the new drug on individuals with no prior history of dengue. With this admission by Sanofi, they have been proven right.”
Undue haste?
A legislative inquiry at the House of Representatives unraveled the conundrum associated with Dengvaxia’s timeline in the country, marked by what one resource person alluded to as misplaced priorities, procedural lapses, and conflicts of interest.

During the first week of December 2015, while on a trip to Paris for the Climate Change Summit, then president Aquino received officials of Sanofi drug company, billed as a follow-up to a previous meeting they held in China, where they discussed the pharmaceutical manufacturer’s dengue vaccine.
According to one resource person at the inquiry, Dr. Anthony Leachon, who was a member of the Dengue Expert Panel convened by the DOH, this was followed by the Food and Drug Administration’s (FDA) approval within the same month (in late December) and then by the purchase request processing around the middle of January 2016, addressed to the Zuellig pharmaceutical company, of two million doses in spite of the absence of Dengvaxia in the listing of the Philippine National Drug Formulary.
The subsequent chain of events followed, with budget allotment getting cleared on January 25, 2016, then the actual purchase order on March 9, and then the actual purchase, until the formal start of the program in April 2016, just before the national election and in spite of misgivings from the scientific community about the lack of a detailed study on the safety of the vaccine’s recipients.

By April 2016, DOH decided to start inoculating the schoolchildren. Senator Gordon pointed out that the guidelines were given only in April “after the vaccine had already been used. We cannot have these people pretending and playing God in our country, and say, ‘let’s put P3.5 billion here, not even Congress can question us because we make the decisions’.”
Garin explained that the Food and Drug Agencies of Mexico and Brazil had already reviewed the safety of the vaccine.
Funding for Dengvaxia not from DOH
Her successor, Dr. Paulyn Rosell-Ubial, revealed to a legislative inquiry that the money used by DOH to procure Dengvaxia was not organically from the Department’s budget for 2016 but disbursed by the Department of Budget and Management scraped together from unobligated funds of other instrumentalities.
Gordon quipped that this PhP3.5-billion transaction has turned the country into “the number one guinea pig in Asia,” especially as there were health officials who conceded that there was no reliable way to determine the extent of data integrity or if the vaccine, indeed, yielded positive outcomes, because many of the children either received only one of the three doses or were unable to complete the three-dose prescription.
The way the program was executed, Gordon said, underscored “an awful lot of questions about this sudden undue haste in providing this dengue vaccine. It might imperil the child or render the vaccinations inutile.”
Advisory, information drive
For her part, Senator Nancy Binay called on the Department of Health and Sanofi to launch a nationwide medical advisory and information drive to reach out to parents whose children have taken the vaccine: “The public – particularly the parents – need to know the circumstances surrounding the vaccine, what symptoms should be monitored, the precautions, indications, contra-indications, side effects and possible adverse effects.”
Binay added, “it is sad that the DOH ignored warnings about the issue of patient safety and research integrity of Dengvaxia. Safety should always be the paramount concern when it comes to any immunization program. We don’t want the warning to the public to come too little, too late. Obviously, there were shortfalls and gaps in the vaccine’s safety profile, and I believe Sanofi is morally and ethically obliged to inform the public about what came out in their clinical tests.”
The immunization program was launched on April 4, 2016. However, according to the Philippine Daily Inquirer, only 493,000 schoolchildren, or less than half the one-million target, were vaccinated in the first round. “Numbers dropped even more to 416,508 and 97,515 for the administering of the second and third doses,” PDI cited DOH Disease Prevention & Control Bureau officer-in-charge Ma. Joyce Ducusin as saying.